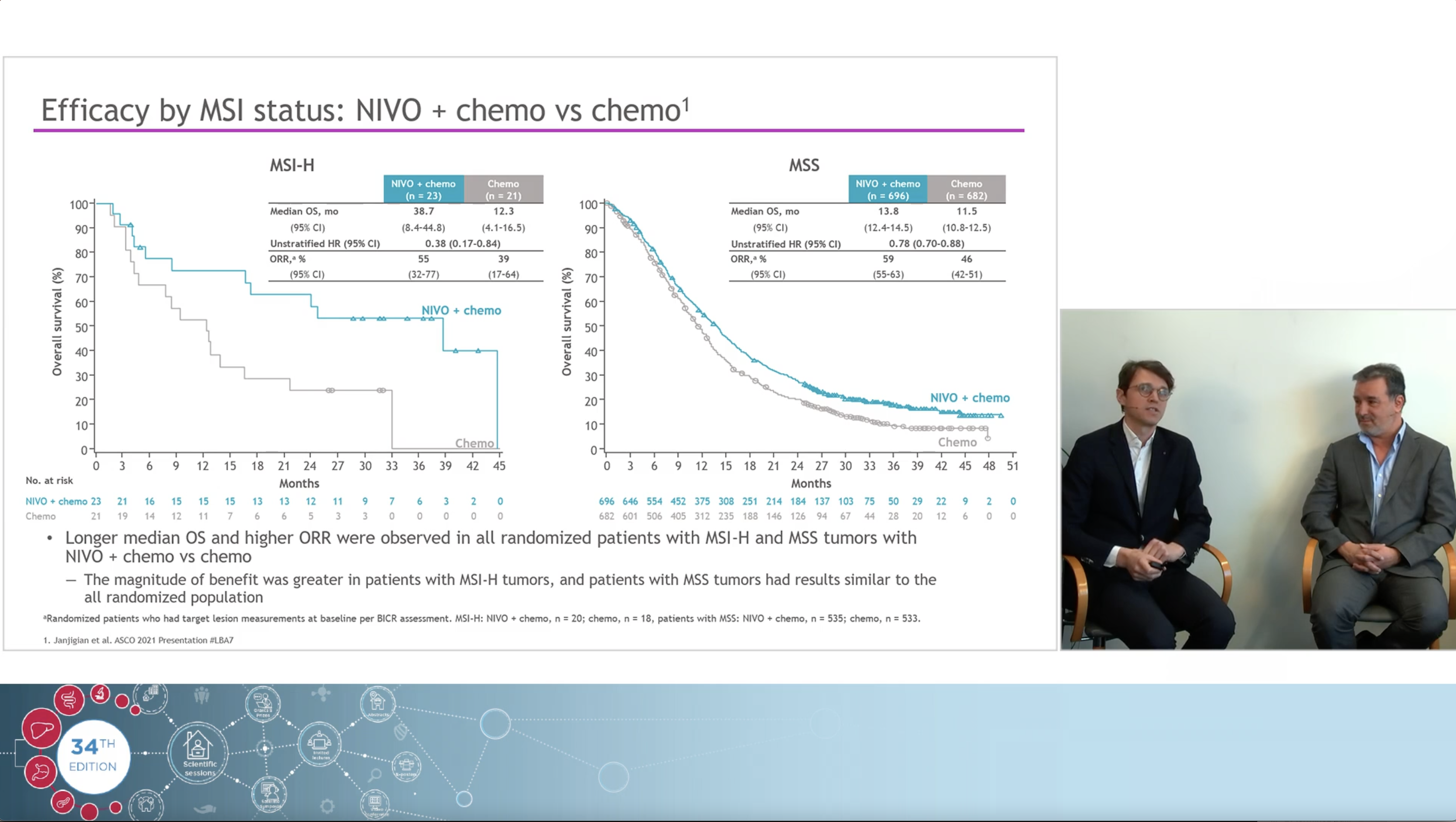
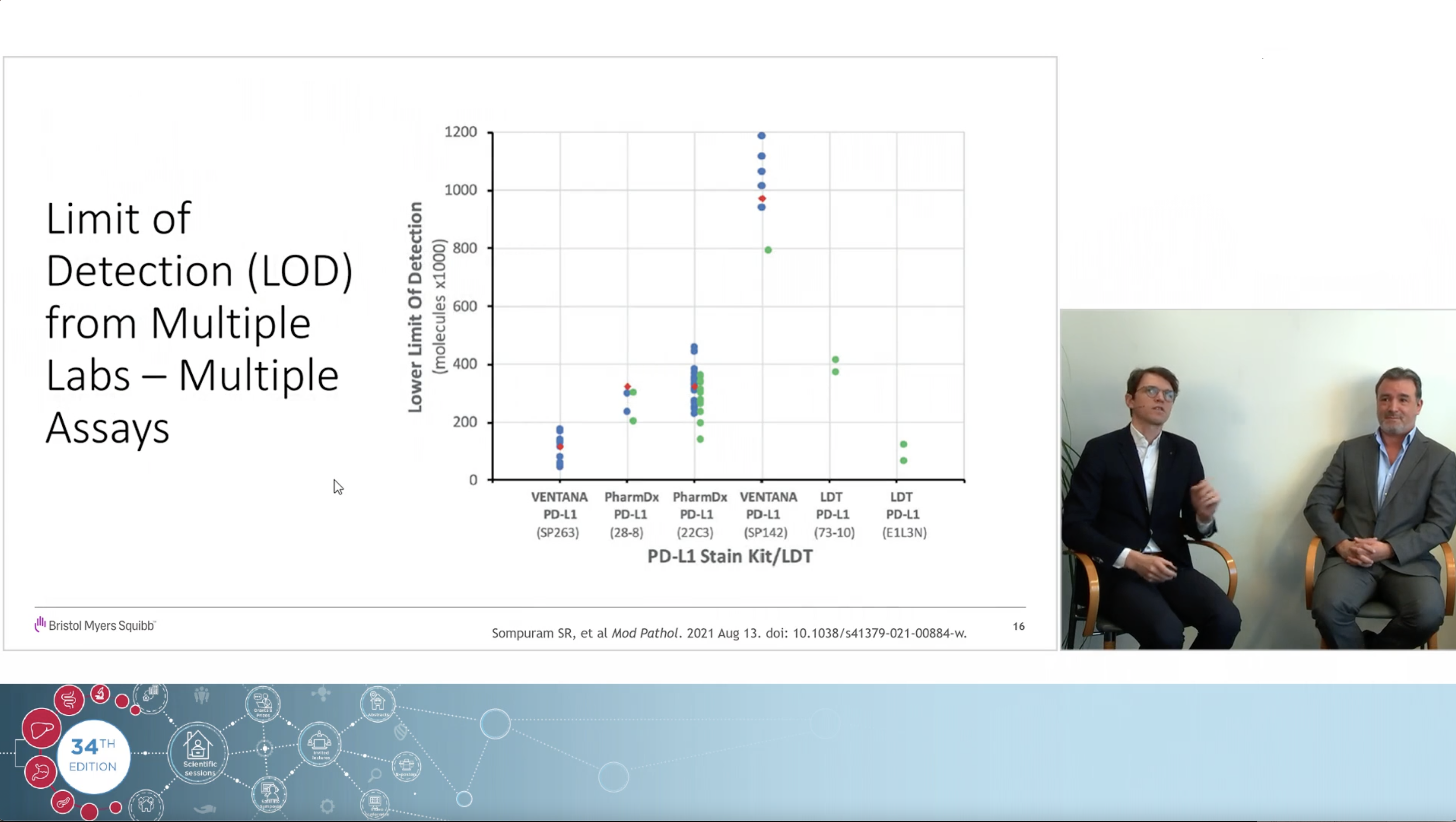
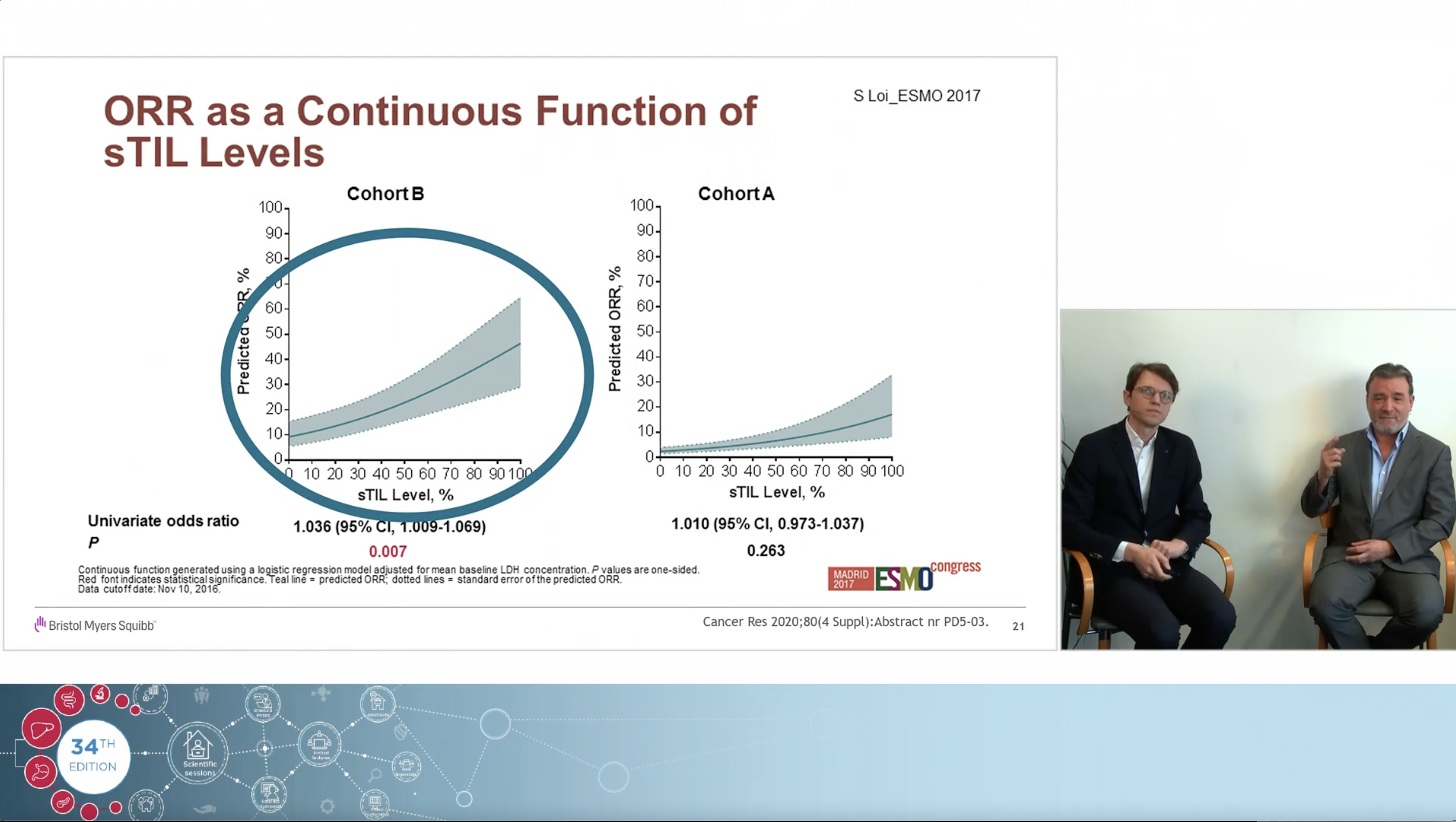
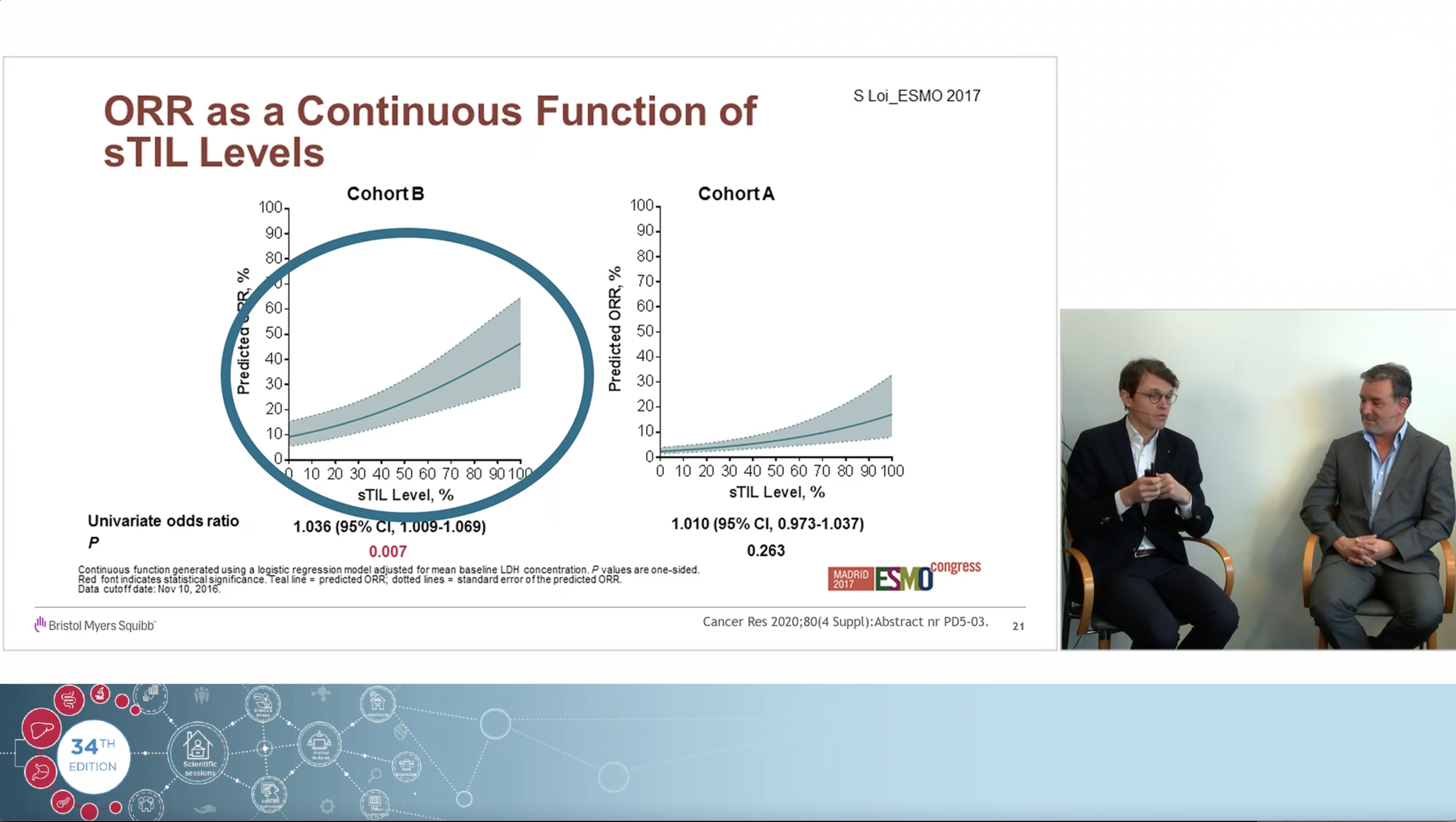
Using a real clinical case as an example, the experts, Dr Jeroen Dekervel (gastro-oncologist, UZ Leuven) and Dr Roberto Salgado (pathologist, GZA Hospital Networks Antwerp), share their views on how immunotherapy changed the treatment landscape in Upper GI cancers and how to navigate the complexities and controversies of PD-L1 CPS testing associated with the arrival of those new indications.
This website is intended for healthcare professionals based in Belgium and Luxembourg.If you are not a healthcare professional in Ireland, click here
You may contact our EU Data Protection Officer at EUDPO@BMS.com to exercise any data privacy rights that you may have, as well as to raise any concerns or questions in relation to the handling of your personal data by Bristol-Myers Squibb Company.