Expert opinion

NEW
Prof. Martin Reck (Germany)
interviewed by Prof. Christophe
Dooms (UZ Leuven) on CheckMate
9LA update at 5 years

Where is the highest unmet
medical need in 1L mNSCLC?
BTOS 2023 BMS symposium
with Dr Deschepper

ECLIPSE webinar series for continuous I-O learning via live peer-to-peer clinical practice exchanges
Product information
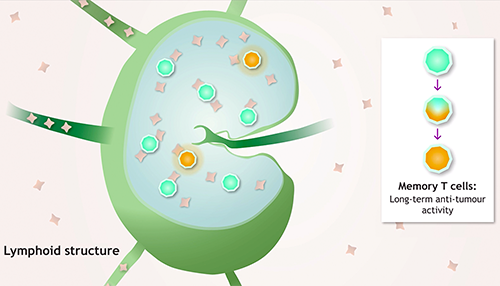
A short video on
the rationale of combining
OPDIVO and YERVOY
in collaboration with
P. Coulie

Dosing scheme
OPDIVO+YERVOY
+ 2 CT cycles
in 1L NSCLC

Practical user guide
based on OPDIVO SmPC
Patient & nurse materials

A diary for your patients treated with
OPDIVO + YERVOY
www.immunooncology.be
An educational website
on immunotherapy for your
nurses and your patients

Patient brochure
with practical information
on immunotherapy

Patient alert card
OPDIVO