OPDIVO as monotherapy is indicated and reimbursed for the adjuvant treatment of adult patients with oesophageal or gastro-oesophageal junction cancer who have residual pathologic disease following prior neoadjuvant chemoradiotherapy.
OPDIVO as monotherapy is indicated and reimbursed for the adjuvant treatment of adult patients with oesophageal or gastro-oesophageal junction cancer who have residual pathologic disease following prior neoadjuvant chemoradiotherapy.
OPDIVO as monotherapy is indicated and reimbursed for the adjuvant treatment of adult patients with oesophageal or gastro-oesophageal junction cancer who have residual pathologic disease following prior neoadjuvant chemoradiotherapy.
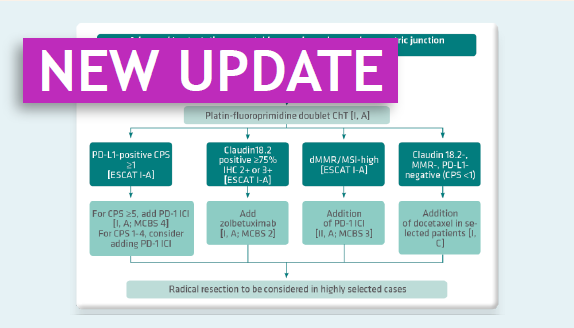
OPDIVO in combination with fluoropyrimidine- and platinum-based combination chemotherapy is indicated and reimbursed for the first-line treatment of adult patients with unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma with tumour cell PD-L1 expression ≥ 1%. Please note, as from 1st September 2023, OPDIVO is no longer reimbursable as 1L treatment if the patient has progressed on a previous line of checkpoint inhibitor
OPDIVO in combination with fluoropyrimidine and platinum-based combination chemo-therapy is indicated and reimbursed for the first-line treatment of adult patients with HER2‑negative advanced or metastatic gastric, gastro-oesophageal junction or oesophageal adenocarcinoma whose tumours express PD-L1 with a combined positive score (CPS) ≥ 5. Please note, as from 1st September 2023, OPDIVO is no longer reimbursable as 1L treatment if the patient has progressed on a previous line of checkpoint inhibitor
OPDIVO in combination with fluoropyrimidine and platinum-based combination chemo-therapy is indicated and reimbursed for the first-line treatment of adult patients with HER2‑negative advanced or metastatic gastric, gastro-oesophageal junction or oesophageal adenocarcinoma whose tumours express PD-L1 with a combined positive score (CPS) ≥ 5. Please note, as from 1st September 2023, OPDIVO is no longer reimbursable as 1L treatment if the patient has progressed on a previous line of checkpoint inhibitor
OPDIVO in combination with fluoropyrimidine and platinum-based combination chemo-therapy is indicated and reimbursed for the first-line treatment of adult patients with HER2-negative advanced or metastatic gastric, gastro-oesophageal junction or oesophageal adenocarcinoma whose tumours express PD-L1 with a combined positive score (CPS) ≥ 5. Please note, as from 1st September 2023, OPDIVO is no longer reimbursable as 1L treatment if the patient has progressed on a previous line of checkpoint inhibitor
OPDIVO as monotherapy is indicated and reimbursed for the treatment of adult patients with unresectable advanced, recurrent or meta-static oesophageal squamous cell carcinoma after prior fluoropyrimidine- and platinum-based combination chemotherapy.


%20banana_2.png)




















2.svg)


